Using Freezing Point Depression to Find Molecular Weight
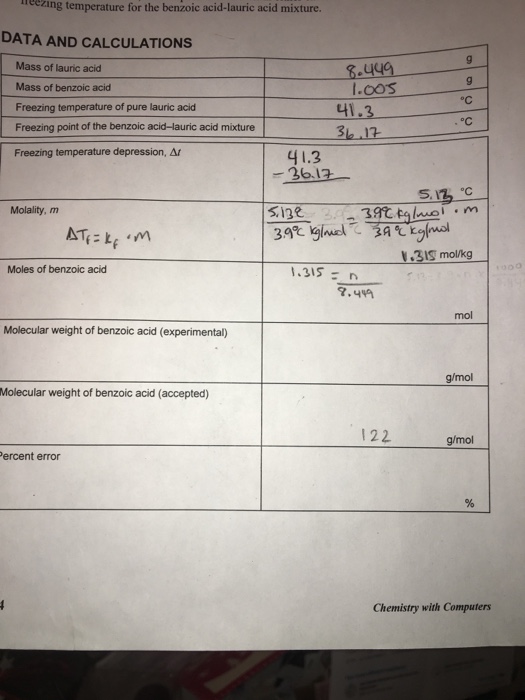
Use the formula D t t 1 - t 2. Calculate molality m in molkg using the formula D t K f m K f 39C-kgmol for lauric acid.
Using Freezing Point Depression To Find Molecular Chegg Com
What are the colligative properties of a solution.
. The freezing point The range was then adjusted to 000 and a depression varies for different pure solvents with second benzene sample is charged in and the Kf of water at 186⁰Cmol and benzene at ΔFp is read0882g of Nonane was dissolved in in 512⁰Cmol. Determine the difference in freezing temperatures D t between the pure lauric acid t 1 and the mixture of lauric acid and benzoic acid t 2. Calculate the molecular weight of the unknown using the molal.
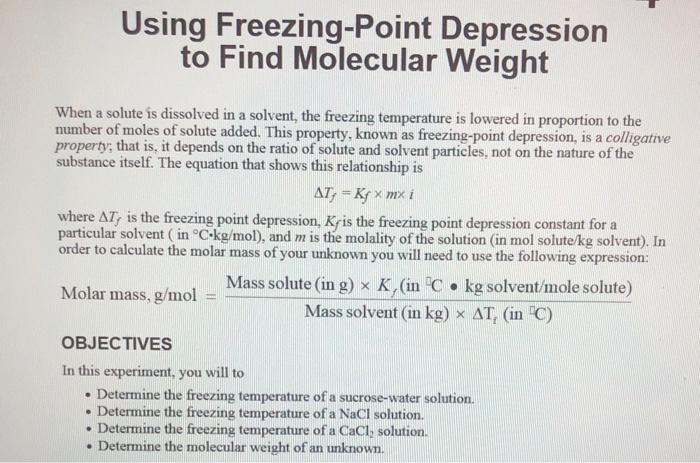
This phenomenon is known as freezing-point depression and the change in temperature is directly related to the molecular weight of the solute. 2 1 1 2 b m y -b y m. Using Freezing-Point Depression to Find Molecular Weight Information.
This experiment is designed to find the identity of an unknown compound by using the phenomenon of freezing-point depression to determine its molecular weight. What is freezing point. That is it depends on the ratio of solute and solvent particles not on the.
LABORATORY REPORT PREPARATION GUIDE MASTER SQL EXPERIMENT 4 Using Freezing Point Depression to Find a Molecular Weight 1. The concentration of the solute will be assessed by. Using Freezing Point Depression to Find Molecular Weight Advanced Chemistry with Vernier 4 - 3 Time Freezing Point 13.
Calculate the freezing point depression of the solution. Molality can be calculated by. When a solute is dissolved in a solvent the freezing temperature is lowered in proportion to the number of moles of solute added.
This property known as freezing-point depression is a. Calculate the molecular weight of benzoic acid. MOLECULAR WEIGHT BY FREEZING POINT DEPRESSION BACKGROUND This experiment demonstrates the use of colligative properties.
429 - 387 39m. The molal freezing point depression constant of benzene is 512 oCmolal. November 2014 Fall freezing point depression.
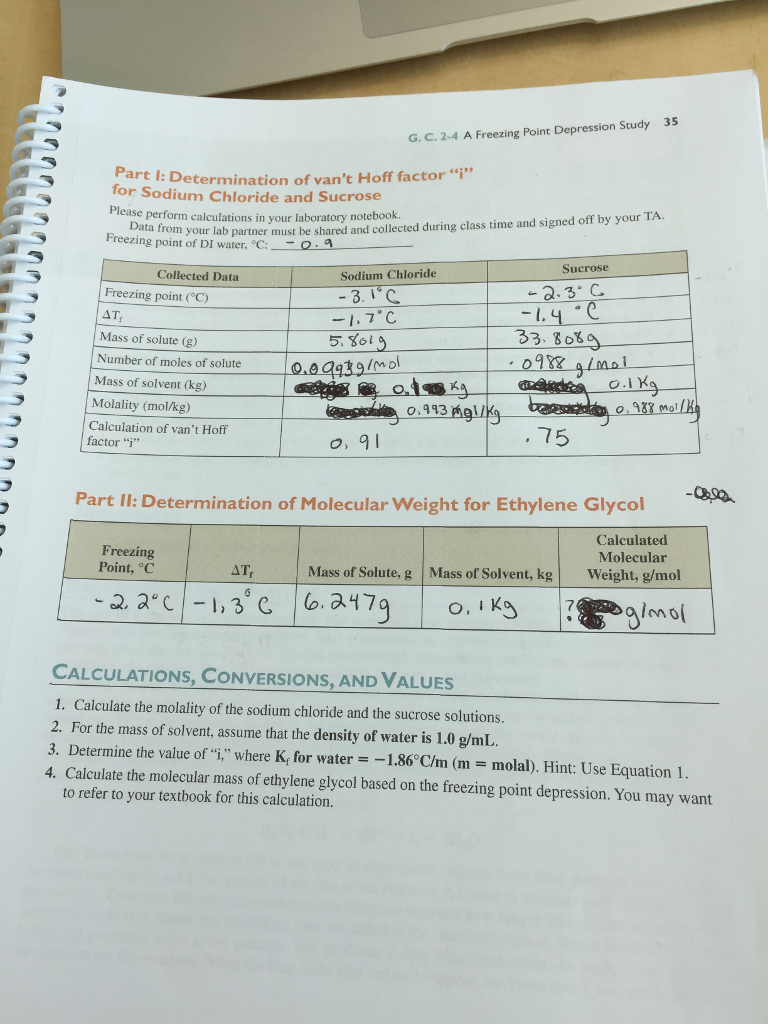
To do this we will use the Logger Pro program to obtain the freezing point of lauric acid and a solution of benzoic acid and lauric acid. The purpose of this lab is to be able to use the freezing point of a substance to find the molecular weight of it. Next the freezing point of a solution prepared from a mixture of an unknown organic acid and lauric acid is measured.
When you are completely done collecting data for the day the freezing temperature of the benzoic acid-lauric acid. This property known as freezing-point depression is. The equation that shows this relationship is.
In this experiment using the formula Δt Kf m. Then well go calculate the freezing point depression of the solution and the molecular weight of benzoic. To do this we will use the Logger Pro program to obtain the freezing point of lauric acid and a solution of benzoic acid and lauric acid.
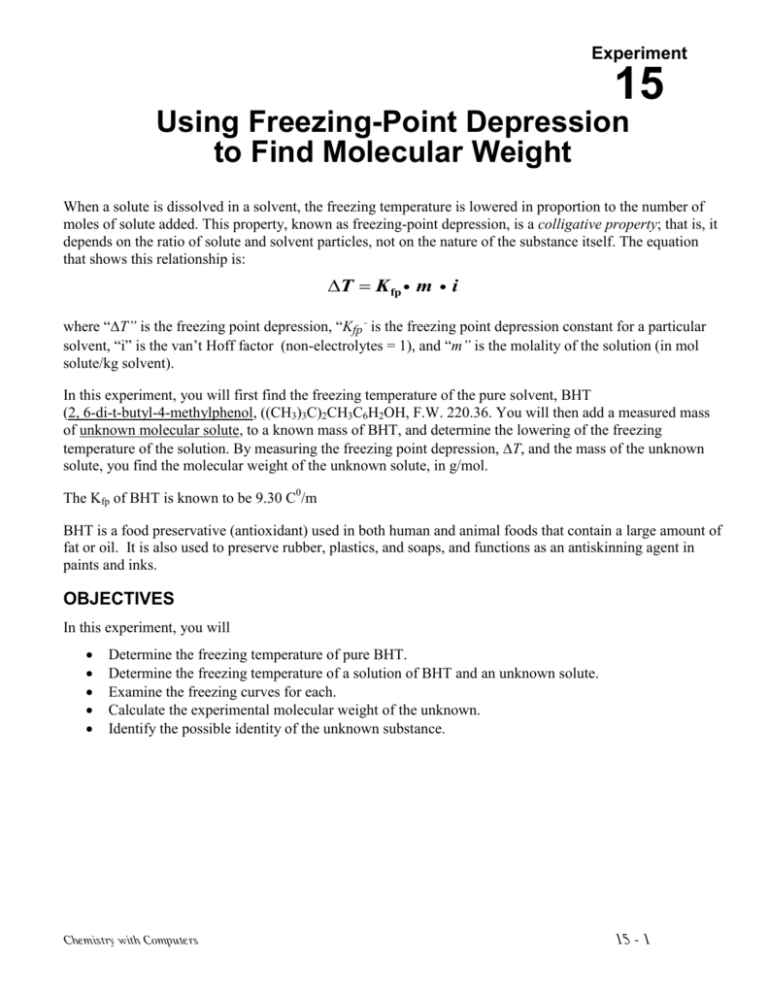
Then well go calculate the freezing point depression of the solution and the molecular weight of benzoic acid. Where Δt is the freezing point depression Kf is the molal freezing point depression. Using Freezing-Point Depression to Find Molecular Weight When a solute is dissolved in a solvent the freezing temperature is lowered in proportion to the number of moles of solute added.
The goal is to measure the molecular weight of a non-volatile solute by determining the concentration dependence of the freezing point depression of a solution. Using Freezing-Point Depression to Find Molecular Weight When a solute is dissolved in a solvent the freezing temperature is lowered in proportion to the number of moles of solute added. Lab 13 Molecular Weight Determination by Freezing Point Depression Revised 8192009 3 To find the intersection of the two lines solve the two equations 4 and 5 simultaneously for y.
M moles of solutekilograms of solvent. This property known as freezing-point depression is a colligative property. T f - T f T f K f m.
What is the molecular weight of the unknown compound. By measuring the masses of the unknown organic acid and lauric acid in the mixture and calculating freezing point depression ΔT and the equations above can be used to find the molecular weight of the unknown acid. When a solute is dissolved in a solvent the freezing temperature is lowered in proportion to the number of moles of solute added.
Save the data file and email it to everyone in your group andor save it on USB drives. Determine the freezing temperature of the pure solvent lauric acid. Using Freezing-Point Depression.
We use the lauric acid molar freezing point constant and the vant Hoff factor for benzoic acid we can calculate the molarity of the solution. What is molality and why does molal concentration affect the colligative properties of a solution. Calculate the molal concentration of the solution using the freezingpoint depression.
Using Freezing-Point Depression to Find Molecular Weight. Using Freezing-Point Depression To Find Molecular Weight Pre-lab questions Name. The equation of freezing-point depression we will be using in this experiment is Δt.
Y m1x b1 4 y m2x b2 5 Solving equation 4 for x gives you 1 1 m y - b x which can be plugged into equation 5 to give you equation 6. Determine the freezing temperature of a mixture of lauric acid and benzoic acid. Freezing point of water 0oC Freezing point depression 0oC - -10oC 10oC View the full answer Transcribed image text.
That is it depends on the ratio of solute and solvent particles not on the. Calculate the freezing point depression of the mixture. Constant 39 degrees Celsiuskgmol m is the molality of the solution and i is the vant Hoff.
The purpose of this lab is to be able to use the freezing point of a substance to find its molecular weight of. The equation that shows this relationship is latexDelta t K_f times mlatex where Δt is the freezing point depression Kf is the freezing point depression constant for a particular solvent 39Ckgmol for lauric acid in this experiment and m is the molality of the solution in mol solutekg solvent. The Kf value for lauric acid is 39Ckgmol was used to get the molality.
Use the Freezing Point Depression equation to determine the molality m of the lauric acid in the test tube containing the mixture. LatexDelta T K_f cdot mlatex where ΔT is the freezing point depression Kf is the freezing point depression constant for a particular solvent 39C-kgmol for lauric acid in this experiment1 and m is the molality of the solution in mol solutekg solvent. To Find Molecular Weight.
Procedure attached as carbon. That is it depends on the ratio of solute and solvent particles not on the. The K f value for lauric acid is 39Ckgmol.
The solvent used must be one of the compounds. Using Freezing- Point Depression to Find Molecular Weight Introduction In this lab we us a colligative property known as freezing point depression in order to find the molecular weight of benzoic acid. This property known as freezing-point depression is a colligative property.

Molar Mass By Freezing Point Depression

Experiment 3 Using Freezing Point Depression To Find Chegg Com
Lab Molecular Weight By Freezing Point Depression

Calculating Molar Mass From Freezing Point Depression Youtube

Determine The Freezing Point Depression Of H 2o In 1 50 M Solution Of C 12 H 22 O 11 Study Com

4 Using Freezing Point Depression To Find Molecular Weight

Solved Omar Prelaboratory Assignment Determination Of Chegg Com

Freezing Point Depression Determination Of The Molar Mass Of An Unknown Substance Laboratory Record Unknown Identifier Homeworklib

Solved Molar Mass Determination Using Freezing Point Chegg Com

Freezing Point Depression Chemistry For Environmental Engineering

Solved How Do I Calculate Molecular Weight Of Ethylene Chegg Com
Solved Experiment 15 Using The Freezing Point Depression Chegg Com

Determination Of Molar Mass By Freezing Point
Chemistry 104 Molecular Weight By Freezing Point Depression

Solved Using Freezing Point Depression To Find Molecular Chegg Com

Molar Mass By Freezing Point Depression Con Pre Laboratory Assignment I The Following Data Were Obtained In Homeworklib

Ak Lectures Freezing Point Depression Example

Calculating Molar Mass From Freezing Point Depression Youtube

Comments
Post a Comment